Curating under quarantine
Project description
Curating under quarantine is a curatorial initiative that seeks to respond to the COVID-19 pandemic in ‘real time,’ while considering what moments will be significant for future Canadians. The initiative aims to:
- Decrease social isolation during the pandemic
- Document technological challenges
- Highlight Canadian innovation and adaptations prompted by the pandemic
- Preserve and share the experiences of the public and the museum community
- Develop and experiment with new methods and methodologies of curation
The team is sensitive to the serious—and often terrible—effects that the pandemic is having on people’s lives. We strive to be self-aware, empathetic, and mindful that other people’s experiences of COVID-19 may not be the same as ours. Contributors are conscious of the tone and the timing of projects. Shared authority is crucial to this initiative; Canadians must be able to share their experiences in their own voices, when and if they want.
The ongoing outcomes of this initiative can be found below.
Project outcomes
Research projects
Archive
The Vaccine Rollout Oral History Archive contains interviews with individuals associated with Canada’s COVID-19 vaccination efforts, including scientists, manufacturers, political and medical authorities, as well as recipients. For more information, please contact: [email protected].

Exhibitions

A Day in the Life
This Google Arts & Culture digital exhibition chronicles the path of the first vial of COVID-19 vaccine—from production to the arm of a Canadian healthcare worker.

Vaccines made in Canada – Part 1: Smallpox – Diphtheria – Tetanus
From the late 1800s to the early 1900s, European and American labs made major advances in disease prevention. They produced antitoxins for illnesses that had plagued mankind for centuries—such as tetanus and diphtheria—and improved the production of smallpox vaccine.

Vaccines made in Canada – Part 2: Influenza – Polio – combined vaccines
In 1918, the First World War was ending, and soldiers were returning home to their families. However, they also brought with them a deadly influenza virus that would eventually claim 50 million lives around the world, many of them young.
From The Channel
Acquisitions
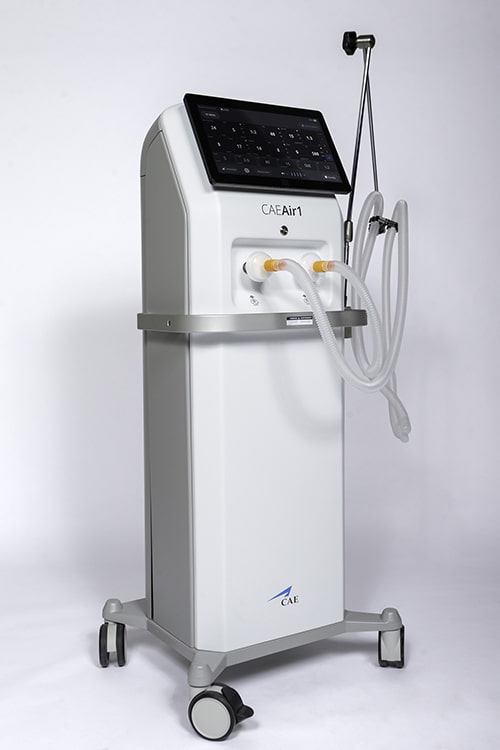
CAE Air1 Ventilator
The CAE Air1 Ventilator is a modern mechanical ventilator built by the world-leading simulation and training company, CAE, in response to the COVID-19 pandemic and the anticipated shortage of life-saving ventilators in Canada. The ventilator was designed very quickly; it was the first such technology to receive certification from Health Canada during the pandemic. Five-hundred CAE employees from incredibly varied backgrounds volunteered to work on the project in various capacities, including in the factory assembling the units. This made-in-Canada ventilator is made up of over 500 parts, sourced from 130 Canadian suppliers. The aerospace industry has been so hard hit by the pandemic; this is one of the few positive stories and lifelines that aerospace companies have had over the past 14 months. As of early spring 2021, 8,200 ventilators have been manufactured to date.

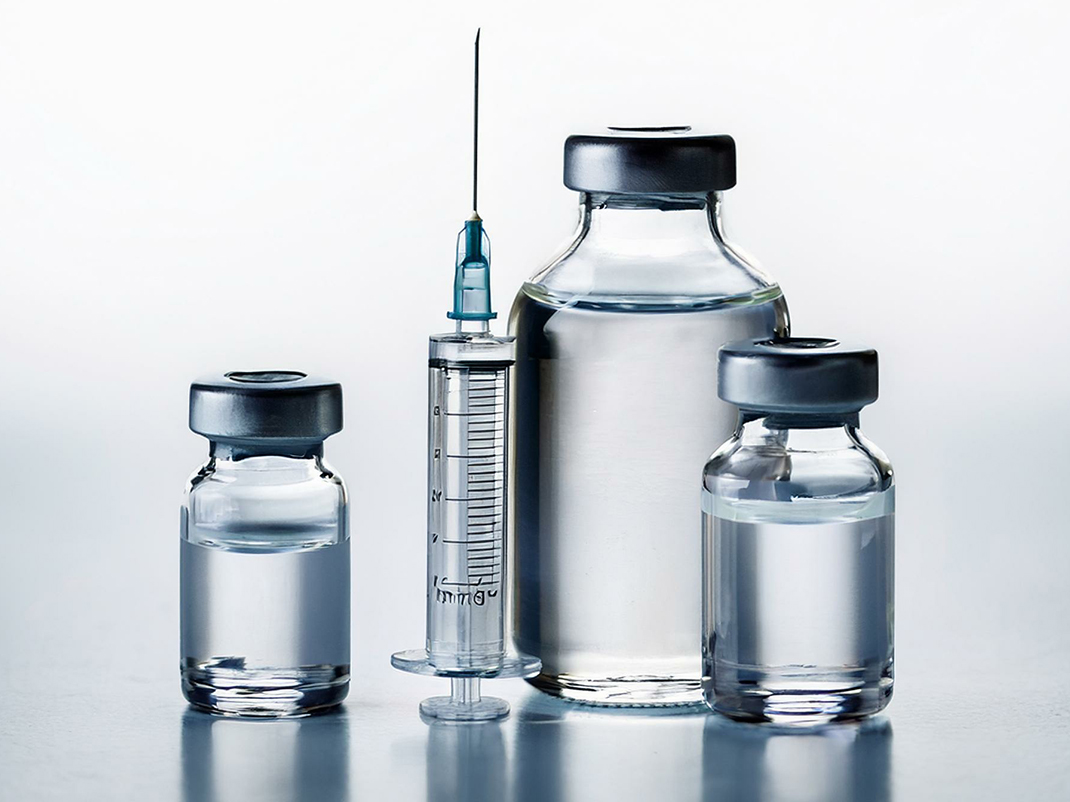
Pfizer-BioNTech COVID-19 Vaccine Vials
On December 14, 2020 and January 4, 2021, the University Health Network administered the contents of these vials to five employees of Toronto’s Rekai Centre; they were the first healthcare workers in Canada to receive the Pfizer-BioNTech COVID-19 vaccine. While this certainly was a significant event in Canada’s response to the pandemic, the vials also connect to critical collaborative research efforts, accessible supply chains, and vast knowledge systems that informed their development, testing, and ultimate mass production. One such example is the Vancouver-based biotechnology company, Acuitas Therapeutics, which developed the nanotechnology—or delivery system based on lipid nanoparticles—that allows the messenger RNA in the Pfizer-BioNTech vaccine to be safely delivered to its target.

General Motors Canada’s 10-millionth mask
The first known case of COVID-19 in Canada dates to January 23, 2020. Within months, the country faced a critical mask shortage. In response to this urgent need, on April 24, 2020, the Federal Minister of Innovation, Science and Industry announced that the government had signed a letter of intent with General Motors Canada which would see the manufacturer produce 10 million medical-grade facemasks by the spring of 2021. Ingenium has acquired the 10 millionth mask, as an example of a crucial piece of personal protective equipment worn throughout the pandemic. The mask’s manufacturing provenance also connects to the effort of many industries, both in Canada and worldwide, to respond to COVID-19 by pivoting their production and work products in order to address new needs and shortages.
Video profiles
While many people are looking forward to forgetting and moving
past the current pandemic, curators and museums around
the world are considering how this moment in time will be remembered.
How can we best document and preserve artifacts from an event
we are living through.
When everything could be an artifact, how do we determine
what will best represent this historical time in the future?
Whose COVID stories are being preserved and whose are at risk of being lost?
How can we capture evidence of a time that everyone is eager to move past.
Curating under quarantine as a curatorial-led initiative
developed by Ingenium. It’s our response to the pandemic and the desire
to make sense of — and preserve — what’s happening around us,
through the lens of science and technology.
I’d like to share a few stories
about one of our most recent pandemic related acquisitions.
Two empty vials of the Pfizer-BioNTech COVID 19 vaccine.
On December 14th, 2020 and January 4th, 2021,
the University Health Network administered the contents of these vials
to five employees of Toronto’s Rekai Center;
they were the first health care workers in Canada
to receive the Pfizer-BioNTech COVID 19 vaccine.
While this was a significant event in Canada’s response
to the pandemic, the vials represent more than just this moment.
Their material culture enables us to share stories of critical,
collaborative research efforts, supply chains and vast knowledge systems
that informed their development, testing and ultimate mass production.
They also serve as tiny windows into the future of science, technology
and medicine.
Researchers have been studying messenger RNA,
or mRNA, since its discovery in the 1960s.
A challenge with this technology is that mRNA can quickly degrade before
it can deliver its message — the RNA script — and be read into proteins in the cell.
The solution to this problem came from advances in nanotechnology,
specifically the lipid nanoparticles that wrap the mRNA like a bubble,
protecting it and allowing it to enter into the cells.
For the Pfizer-BioNTech COVID 19 vaccine,
a Vancouver based biotechnology company called the Acuitas Therapeutics
developed the lipid nanoparticles that allow this vaccine
to work. When the COVID 19 pandemic began,
Canada did not have a facility that could be quickly retooled
to produce viral vector vaccines or mRNA vaccines.
Canada’s limited domestic vaccine manufacturing capability
meant that it had to completely depend on global supply chains
and foreign sources for doses. To shepherd vaccines into Canada,
many networks of knowledge, systems of transport and levels of government
had to work together to facilitate their procurement and distribution.
These vials were produced at a Corning facility in upstate New York.
Corning is one of a handful of firms
that makes glass vials for the pharmaceutical industry.
Vials that stored drugs or vaccines, such as these, must meet
extremely high safety standards;
they must be shatterproof and resistant to extreme temperatures.
These vials are made of glass, which is a relatively new
pharmaceutical product from Corning.
In 2011, Corning began developing Valor Glass
as part of their research into improving medical vials.
They experimented with different additives and found that adding new ingredients
to the silica and removing
standard elements made for stronger pharmaceutical glass. From New York state,
these vials were shipped to Pfizer BioNTech’s production facility in Belgium.
There, the vials were sent through an intricate and automated
vaccine filling machine where they were washed and sterilized,
and filled with the Pfizer-BioNTech’s COVID 19 vaccine.
The machine sealed them and prepare them for shipment.
Alongside orders for other countries,
the vials destined for Canada were carefully loaded
onto a United Parcel Service airplane on December 12th, 2020.
After stops in Germany and the United States,
the shipment finally landed at the Hamilton International Airport
the evening of December 13th, 2020.
The University Health Network was selected by the province
to oversee the vaccine program in Toronto.
Jin Huh, the network’s senior director of pharmacy, received
the 585 vials as part of the first delivery at 9:40 a.m.
on Monday, December 14th, 2020. In a carefully orchestrated workflow,
he quickly moved the vials through the building,
bringing them to Kelly Lalog, a registered pharmacy technician,
who placed them in the -70 Celsius freezer.
Shortly thereafter, Booth Rumsey, the pharmacy operations technician
supervisor at Princess Margaret Hospital, prepared the vaccines.
She inspected the vials, flipped off the deep purple dust
cover cap, inverted them and inserted a sterile syringe,
which diluted the vaccine with 1.8 millilitres of sodium chloride solution.
After a final inversion to keep the vaccine suspended, and inspection,
she prepared five syringes each with 0.3 millilitres of the vaccine.
A staff member then took the five prepared syringes to the vaccine clinic
at the network’s Michener Institute of Education.
At 12:01 p.m. Tamara Dus, a registered nurse, sat
down to administer
the vaccines to the first five long term care workers to receive the jab in Canada.
The University Health Network set aside a second vial, so that they could guarantee
these frontline workers would receive their second dose in three weeks time.
After the second Ontario-wide stay-at-home order was lifted,
the network shipped these vials to Ingenium
so we could preserve them as part of the national collection.
Since then, these vials have informed an oral history research project
that explores the vaccine rollout in Canada.
Jennifer Fawcett, a researcher
for Ingenium, led this project over the summer of 2021.
Here’s part of her interview with Dr.
Pieter Cullis, Biochemistry and Molecular Biology
Professor at UBC and co-founder of Acuitas Therapeutics.
In this short clip, Dr.
Cullis shares his thoughts on the future of mRNA technology.
– It’s going to revolutionize medicine, really. And then you can see
this happening already. Because you can, you can make any protein you want,
you can edit a protein,
and you can silence the protein. That really covers most human diseases
if you can do any one of those three. There’s a bit of a gold rush going on in the field
at the moment, because so many previously untreatable diseases …
we have a new way to attack them.
While contemporary collecting is not new for museums,
the scope and scale of the COVID 19 pandemic pose unique challenges
for curators. Over the coming years,
we will endeavour to document the pandemic, remaining mindful of its potential
legacies, discrepancies, and unanticipated impacts on society and technology.
Collaborations
Project lead

Emily Gann, PhD
Director, Curatorial Division
Areas of expertise:
Public transportation; Railway technologies and networks; Automobile history in Canada; Gender and technology; Technology, built environment, and access